Apple’s Sleep Apnea feature for Apple Watch has obtained official approval from the US Food and Drug Administration (FDA).
Health Canada, the federal department regulating healthcare products in Canada, licenses the use of the Sleep Apnea feature in the country’s compatible smartwatch. The license was issued on September 26.
The sleep problem detection feature can only be found in the Apple Watch Series 9, Apple Watch Series 10, and Apple Watch Ultra 2. This feature uses device accelerometers to alert its users when they have sleep problems.
Sleep is an important area of health as it impacts a person’s overall physical and mental wellbeing. Sleep apnea is a prevalent disorder in which breathing momentarily stops during sleep, preventing the body from getting enough oxygen.
The condition is estimated to impact more than 1 billion people worldwide, and in most cases, goes undiagnosed. If left untreated, it can have important health consequences over time, including increased risk of hypertension, type 2 diabetes, and cardiac issues.
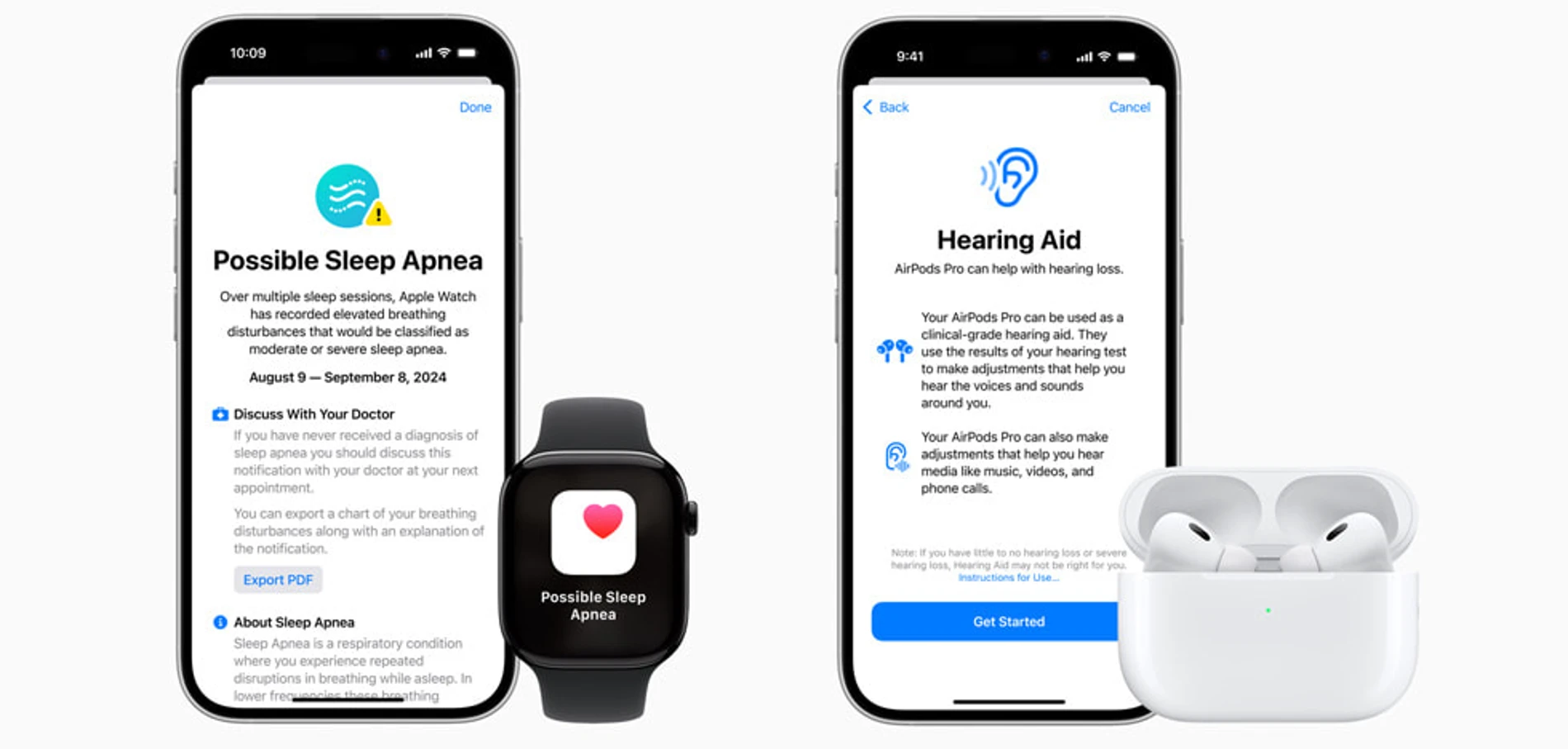
Breathing Disturbances is an innovative new Apple Watch metric that uses the accelerometer to detect small movements at the wrist associated with interruptions to normal respiratory patterns during sleep.
Every 30 days, Apple Watch will analyze breathing disturbance data and notify users if it shows consistent signs of moderate to severe sleep apnea so they can speak to their doctor about next steps, including potential diagnosis and treatment.
Because overall quality of sleep is important, Breathing Disturbances can also be used to assess restfulness of sleep. Breathing Disturbances can be influenced by alcohol, medications, sleep position, and more.
Users can view their nightly Breathing Disturbances in the Health app, where they are classified as elevated or not elevated, and can be viewed over a one-month, six-month, or one-year period.



