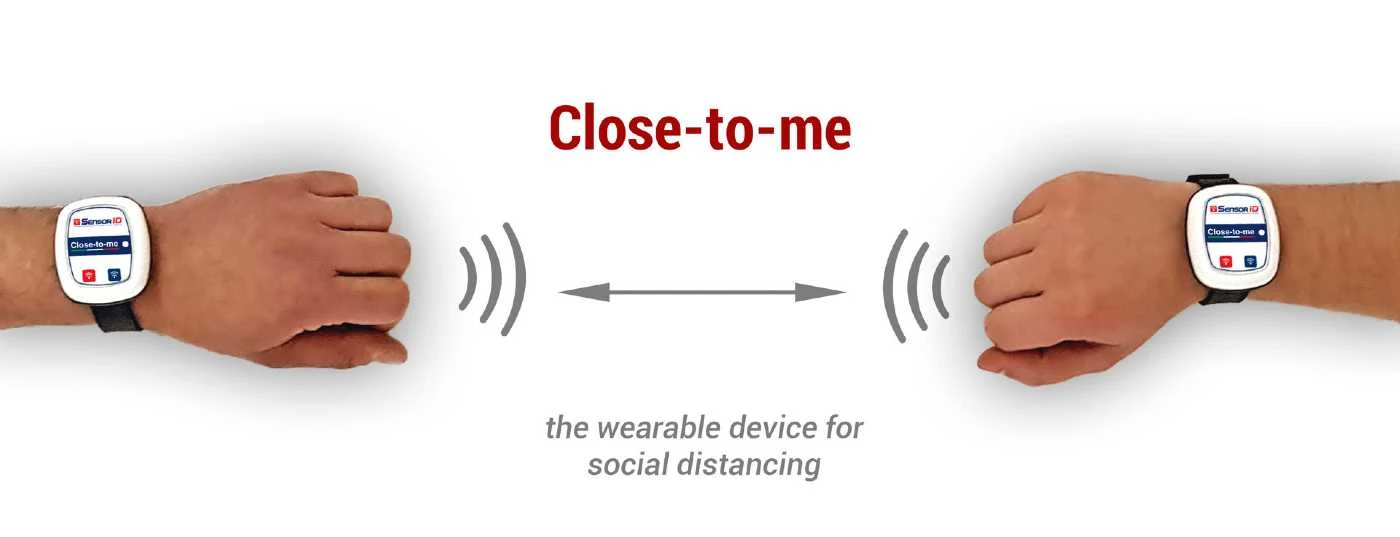
Italian companies Partitalia and SensorID have launched “Close-to-me” and “Vita”, two wearable personal safety devices in the time of Covid-19.
“Close-to-me” is the device that, worn by two or more people present in the same room, monitors “social distancing” based on a variable distance that can be set according to directives and regulations.
Specifically, “Close-to-me” functions in the following way: based on radio frequency technology, it creates a non-invasive, low frequency radio bubble around the person. A sound and vibration warns wearers when the set distance is not respected.
Moreover, through simple adaptations the device can be used to control accesses, detect presences and pay the company canteen.
Alfredo Salvatore, CEO of Sensor ID, engineering company that designed the technology for Partitalia, describes the new product which is already in demand in view of the imminent re-opening of businesses:
“Close-to-me can be personalised and purchased either as a wristband or key-ring: it is non-invasive, designed above all to simplify procedures involved in re-opening businesses and can be implemented easily and rapidly.”
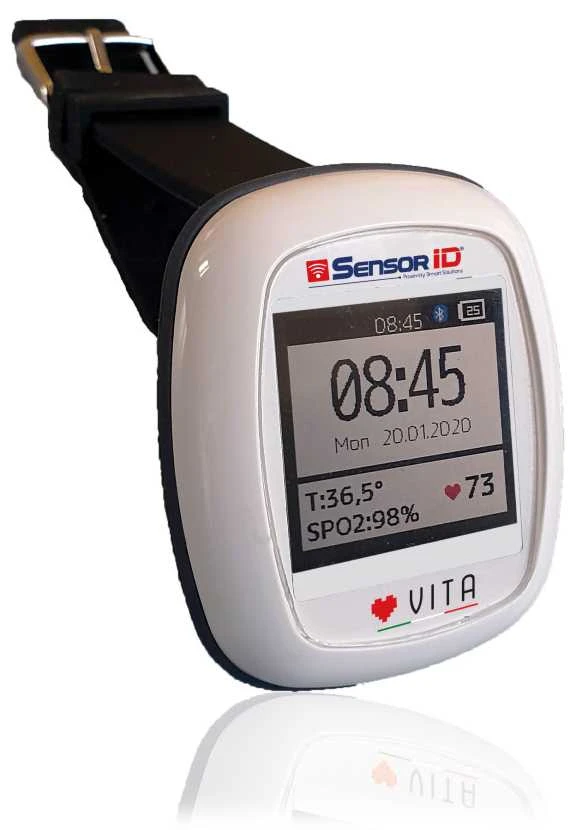
The “Vita” wearable device has been designed to constantly monitor vital parameters in patients that can be treated using telemedicine, in all the cases where it is deemed indispensable to identify a possible contagion.
Technologically, the new frontier of NBloT telecommunications that simplifies communication between objects has been applied in the “Vita” project. Another important characteristic is the improved efficiency of the battery, with a life of more than two weeks.
Additionally, what makes it a tool on the front line for health are the sensors that meter heart beat, oxygen saturation – a fundamental parameter for Covid-19 -, skin temperature and electrocardiogram.
Thanks to these characteristics, besides telemedicine for long-term patients, the wearable device can be used also to monitor lone workers. Therefore, in the time of Covid-19, the device is useful in the case of patients discharged from hospital and subject to remote monitoring, but also in all the other cases of remote monitoring.
The device, which will undergo meticulous clinical experimentation scheduled to start in the next few days, will be certified as a medical device in September 2020.